
EPFLSTILOBMarcel LeuteneggerAbout
Dual-color TIRF fluctuation spectroscopy
Fluorescence spectroscopy in the evanescent field
 |
EPFLSTILOBMarcel LeuteneggerAbout Dual-color TIRF fluctuation spectroscopy Fluorescence spectroscopy in the evanescent field |
|---|

We present the development and first application of a novel dual-color total internal reflection fluorescence (TIRF) instrument [a,b] for single-molecule coincidence analysis and fluorescence fluctuation spectroscopy (FFS). As a performance analysis, we measured a synthetic DNA binding assay, demonstrating this dual-color TIR-FFS approach to be a suitable method for measuring coincidence assays such as biochemical binding, fusion or signal transduction at solid/liquid interfaces. Due to the very high numerical aperture of the epi-illumination configuration, our instrument provides a very high fluorescence collection efficiency resulting in a two- to three-fold increase in molecular brightness as compared to conventional confocal FFS. Further improvements were achieved through global analysis of the spectroscopic data.
 |
 |
|---|---|
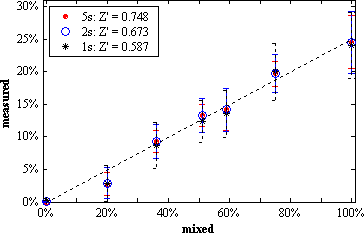
| Figure 1: Photograph of our dual-color TIR-FFS setup. | Figure 2: Measured versus mixed fraction of double-stranded DNA. |
Figure 2 compares the measured versus the mixed fraction of double-stranded DNA labeled with Alexa488 and Cy5. The overlap of the sampling volumes was estimated to 65%, their volumes to 0.2fl. Only about 35% of the dsDNA did not dissociate, resulting in a measured fraction of at most 25%. Inset: measurement times and statistical relevance for the dual-color fluorescence intensity fluctuation analysis (2D-FIDA). A Z' > 0.5 is considered as sufficient for coincidence studies.
With our TIRF microscope, we investigated the insertion of G-protein coupled odorant receptors into supported lipid bilayers. We applied the in vitro synthesis protocol developed by Rudolf Robelek et al. [c] for vectorial insertion of olfactory proteins into an artifical membrane and studied the directonality and density of the receptor insertion as well as their spatial distribution and mobility in the membrane [d].
 |
 |
|---|---|
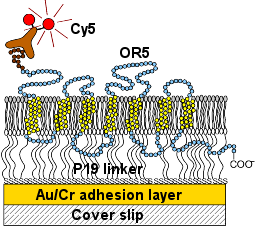
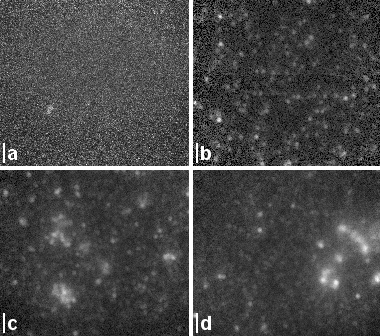
| Figure 3: Membrane assembly with incorporated receptor OR5. The lipid bilayer consists of a supported DMPE monolayer and a floating lipid layer from spread PC vesicles. The OR5 receptor was expressed with a VSV affinity tag at the N' terminus. This tag was immunolabeled with a fluorescently labeled antibody after the in situ expression. | Figure 4: TIRF membrane images versus expression time of (b) 30min, (c) 60min and (d) 90min. (a) Negative control after 60min incorporation of OR5 with a VSV tag at the C' terminus. If the protein is inserted vectorially, the C' terminus is inaccessible for the immunolabel. Scale bars: 2um. |
We would like to acknowledge all supporters of this work. In particular, we would like to thank
We received funds by the Swiss National Science Foundation (SNSF #200021-103333). The functional synthetic membranes (FuSyMEM) project was funded by the European comission.
© 2011 École Polytechnique Fédérale de Lausanne